Schedule your consultation today
Serving Brentwood & the Greater Nashville, TN Area
DERMAL FILLERS • Brentwood, TN
performed by a FELLOWSHIP-TRAINED FACIAL PLASTIC & RECONSTRUCTIVE SURGEON
Dermal Fillers
(I.E. JUVÉDERM®, RESTYLANE®)
Dermal fillers are an injectable treatment that consists of hyaluronic acid, which is a substance that is produced naturally in our bodies. Found mostly in our skin, eyes, and connective tissues, hyaluronic acid acts to retain water and moisture, which is responsible for the firmness and vibrancy of youthful skin. Our bodies' production of hyaluronic acid naturally slows down as we age, which becomes apparent when wrinkles and folds start to appear on the skin.
Versatile, safe, fast-acting, and long-lasting, dermal fillers are clinically proven to be effective for the following:
- treating moderate to severe facial wrinkles and folds below the forehead and eyes
- restoring lost volume to the cheeks and chin
- sculpting the cheek, nose, and chin to enhance facial harmony and proportion (as a possible alternative to cheek, nose, and chin enhancement surgery)
- lip augmentation
- treating depressed acne scars
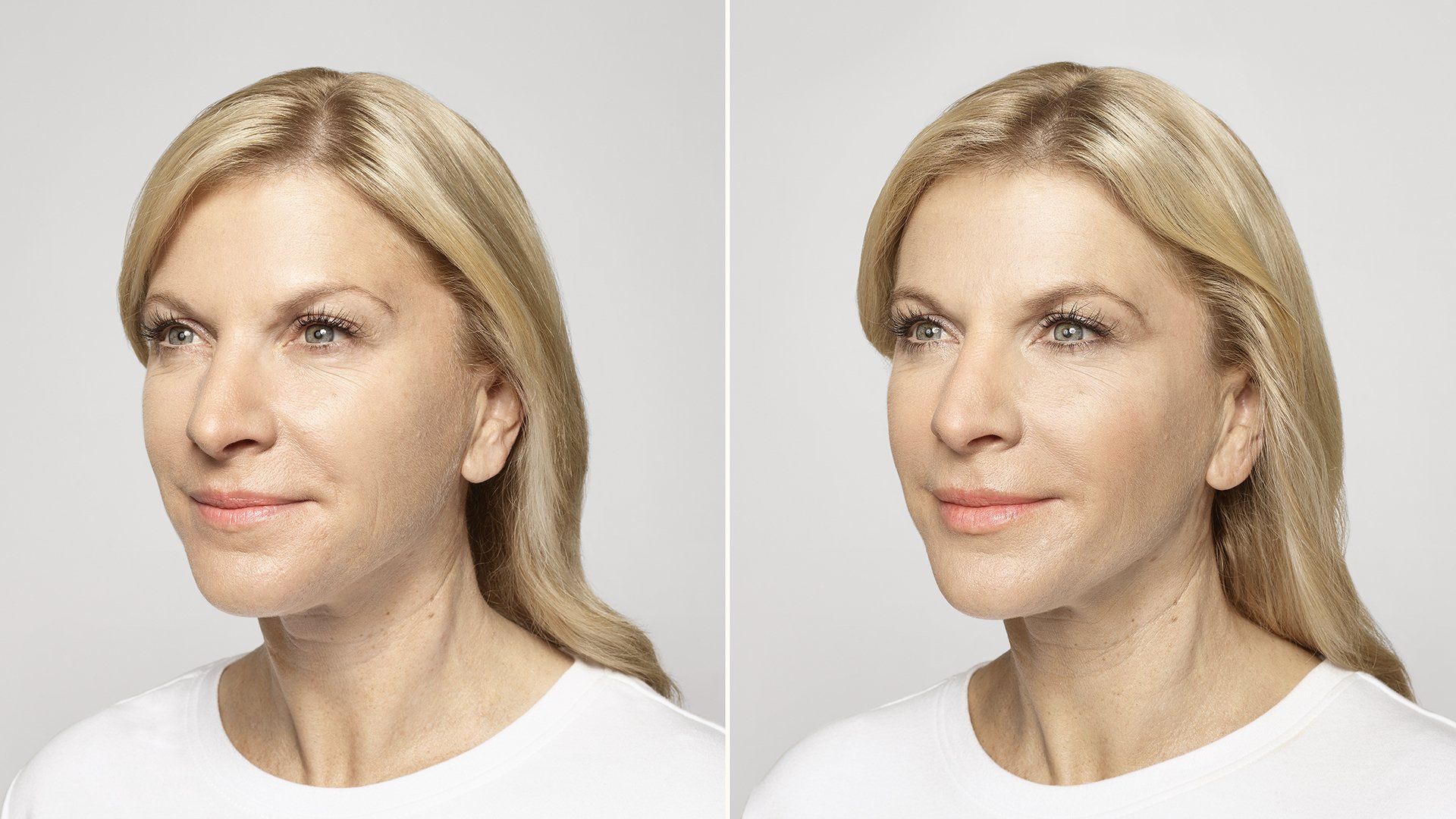
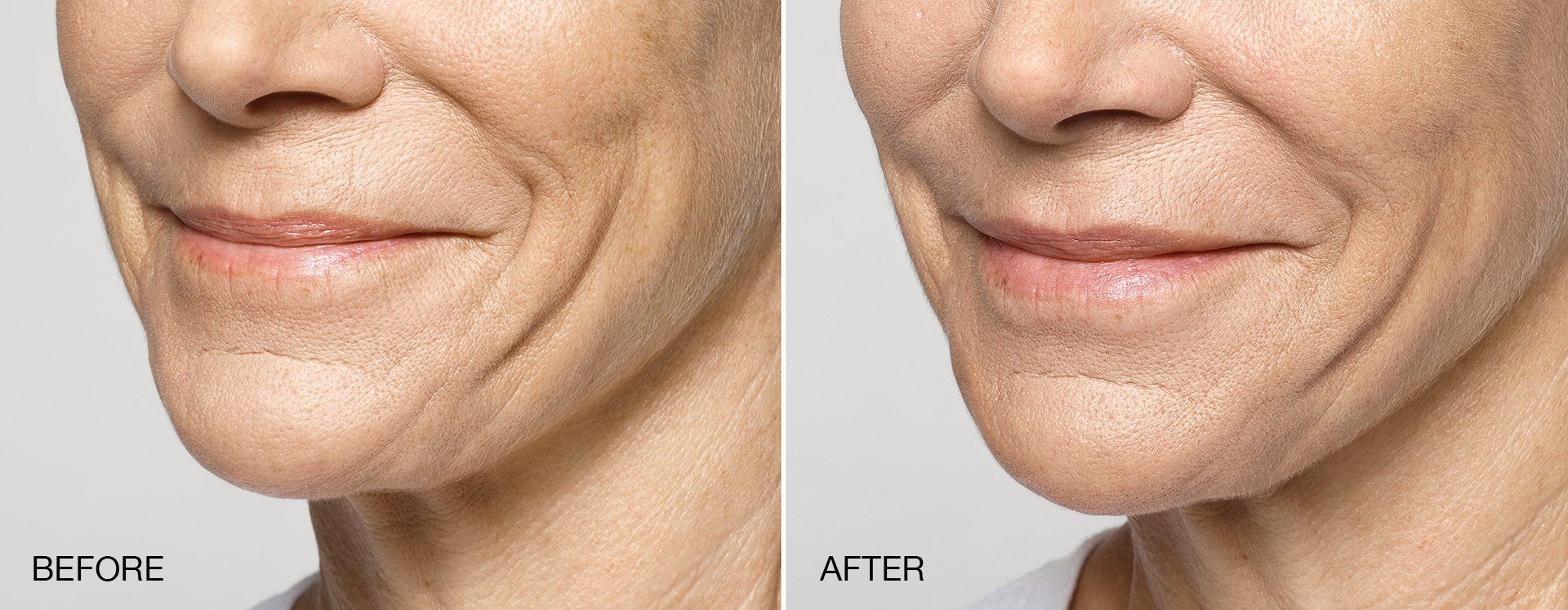
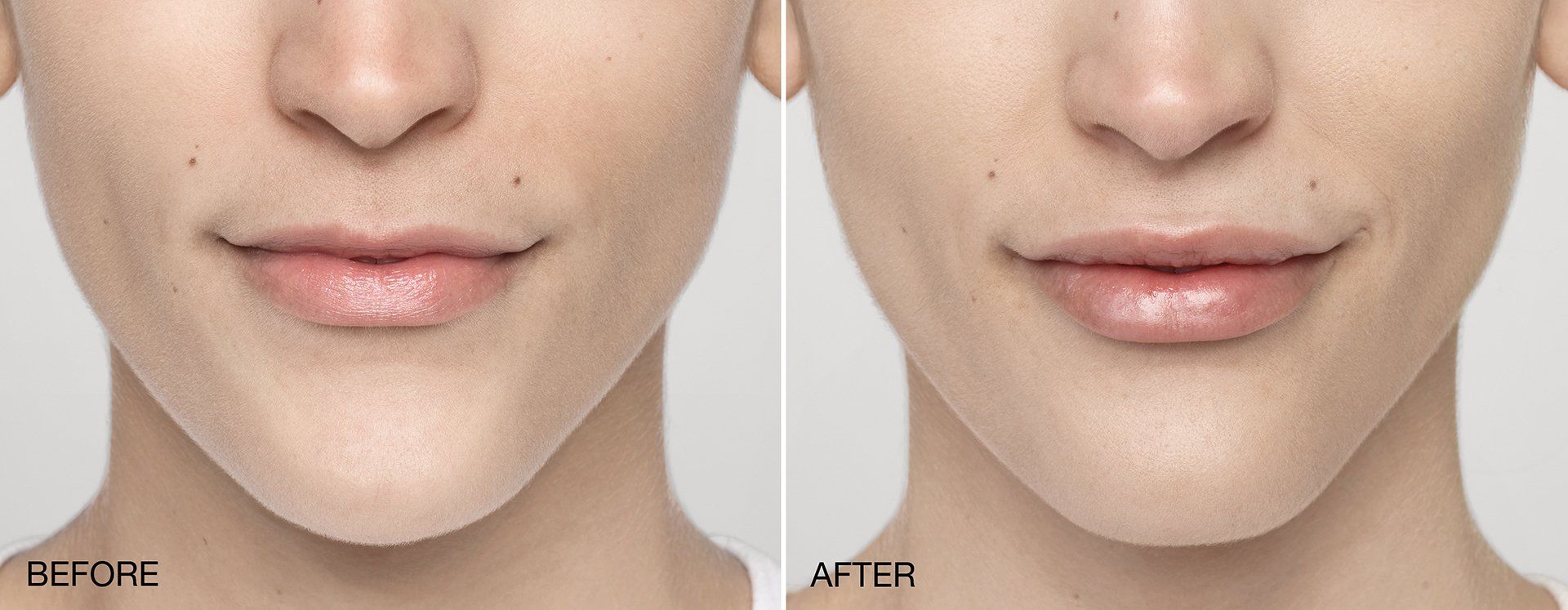
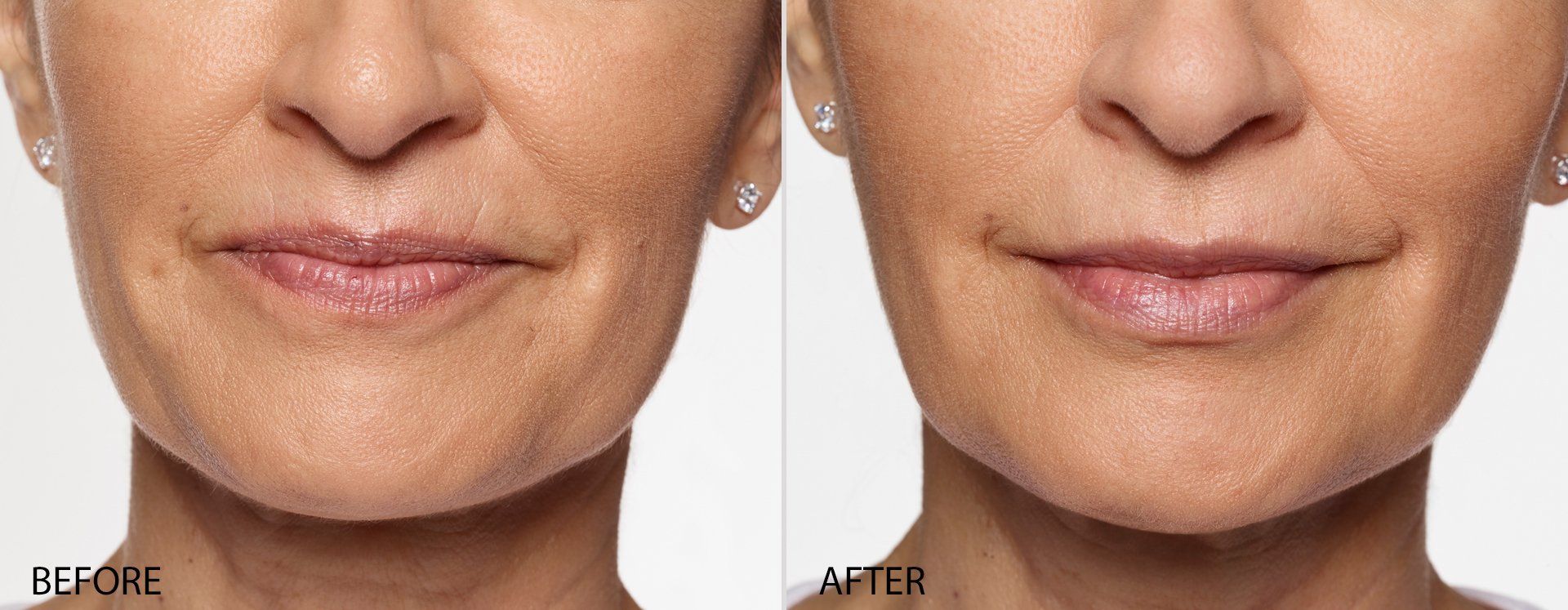
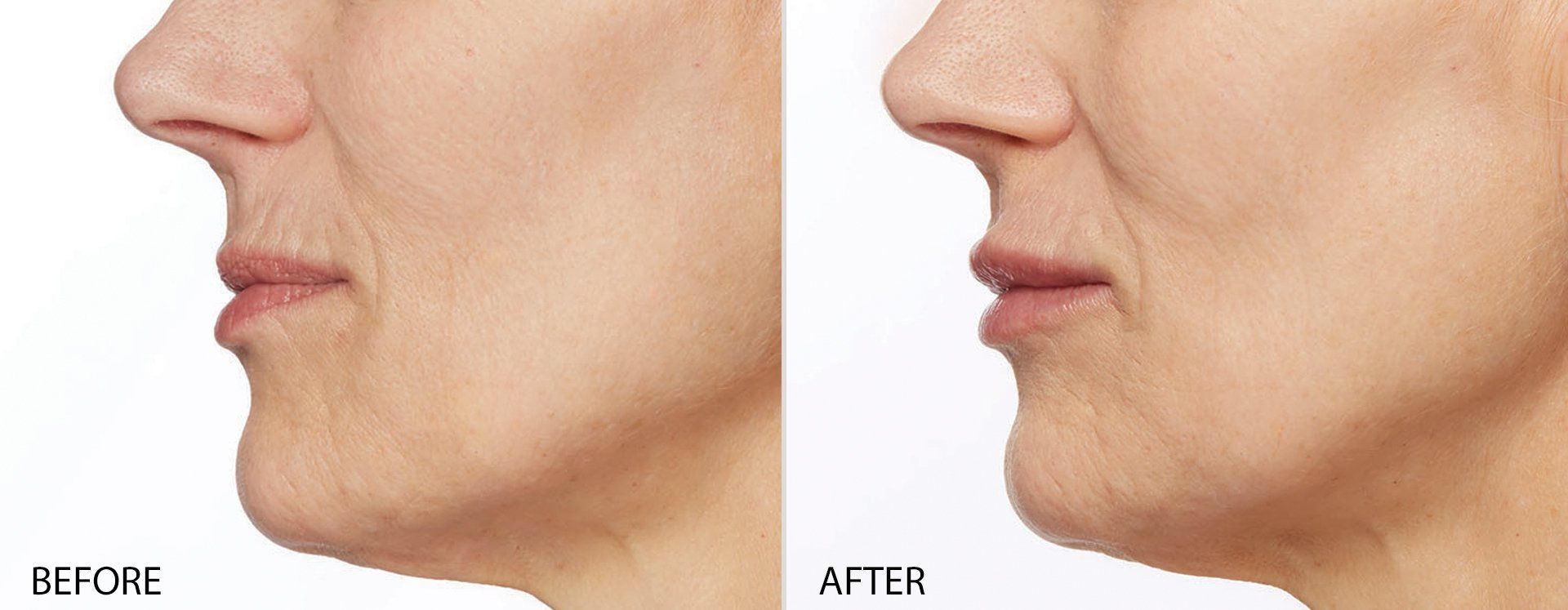
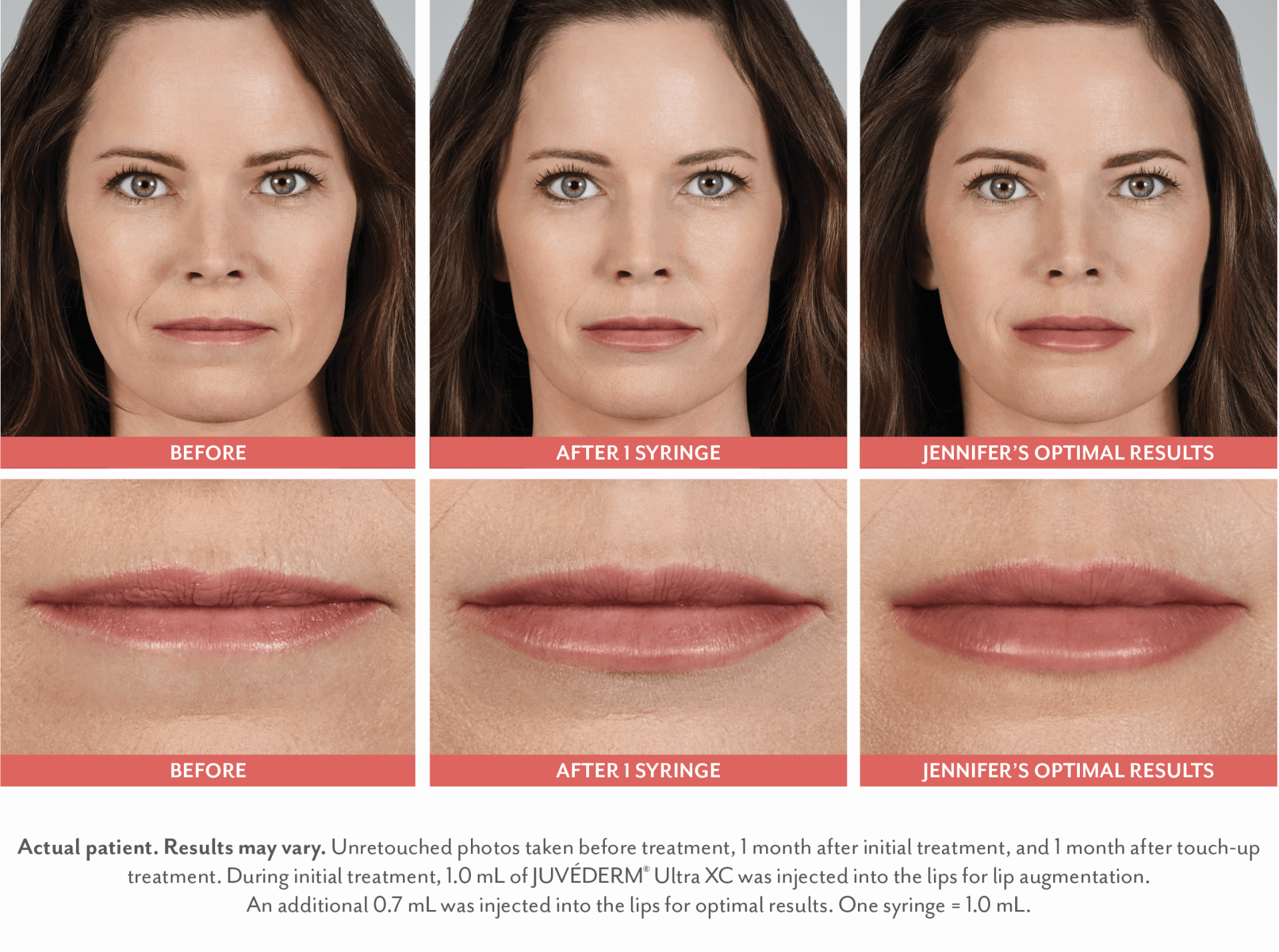
FILLERs Before & After Gallery
Slide title
Write your caption hereButton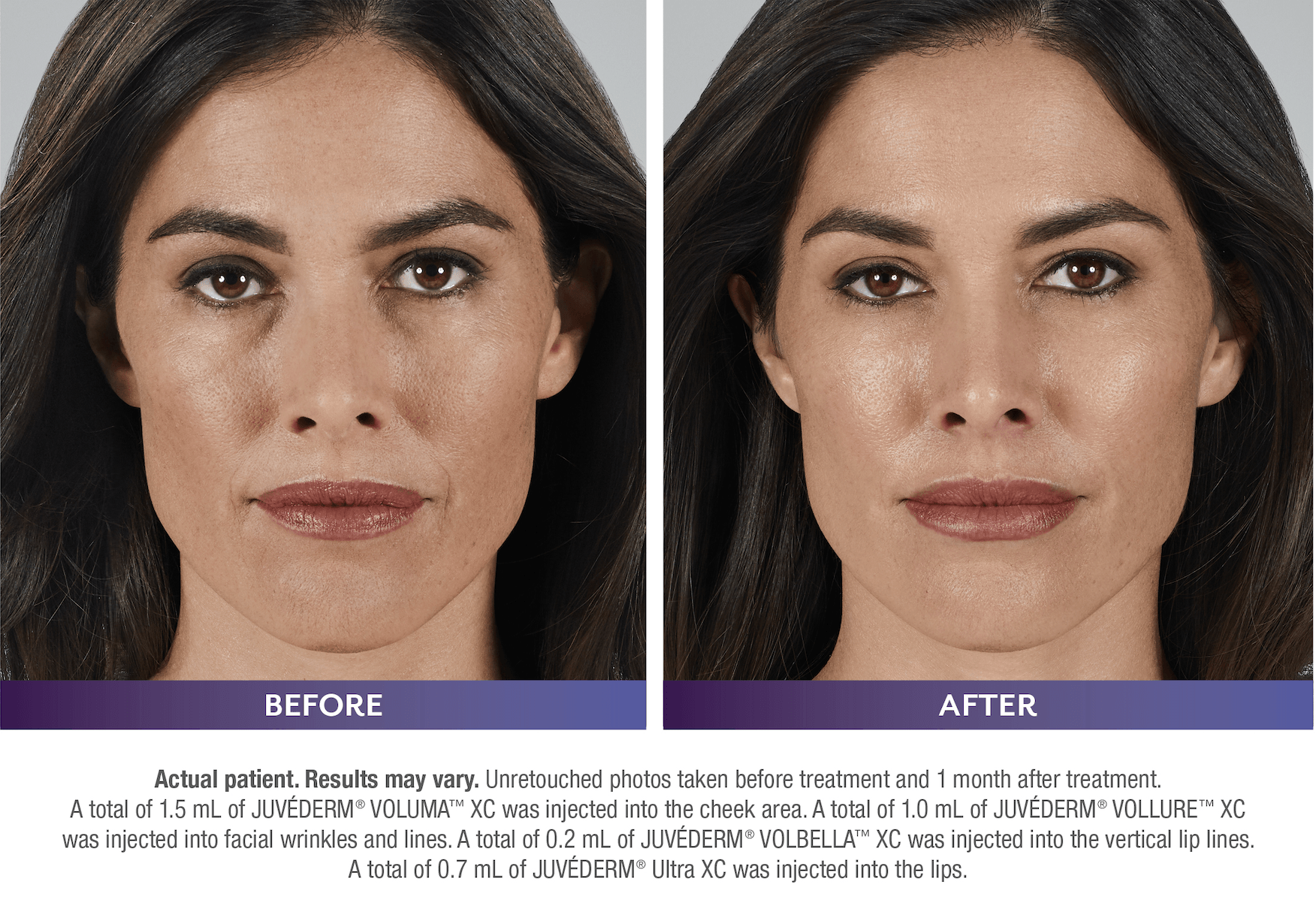
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton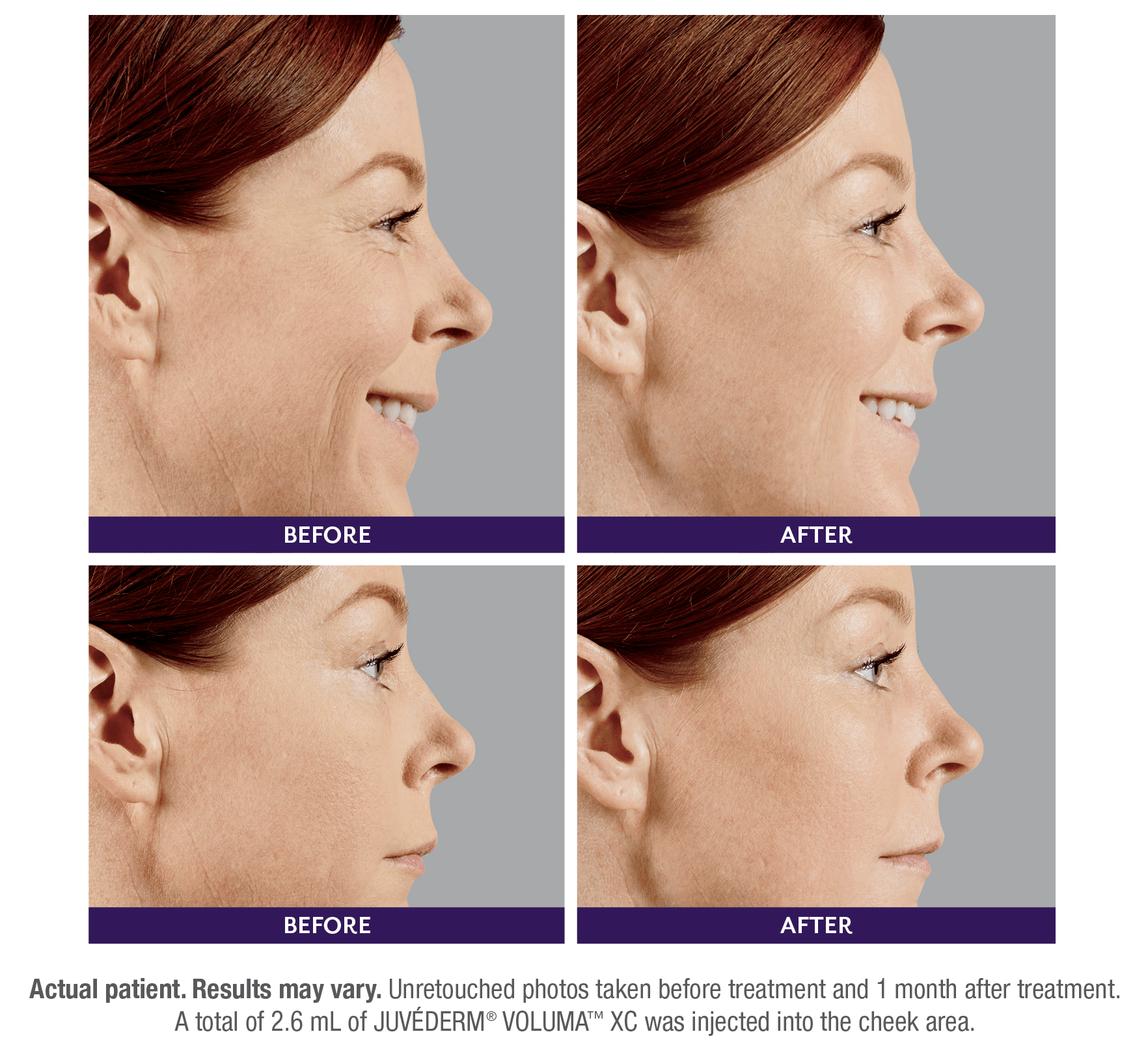
Slide title
Write your caption hereButton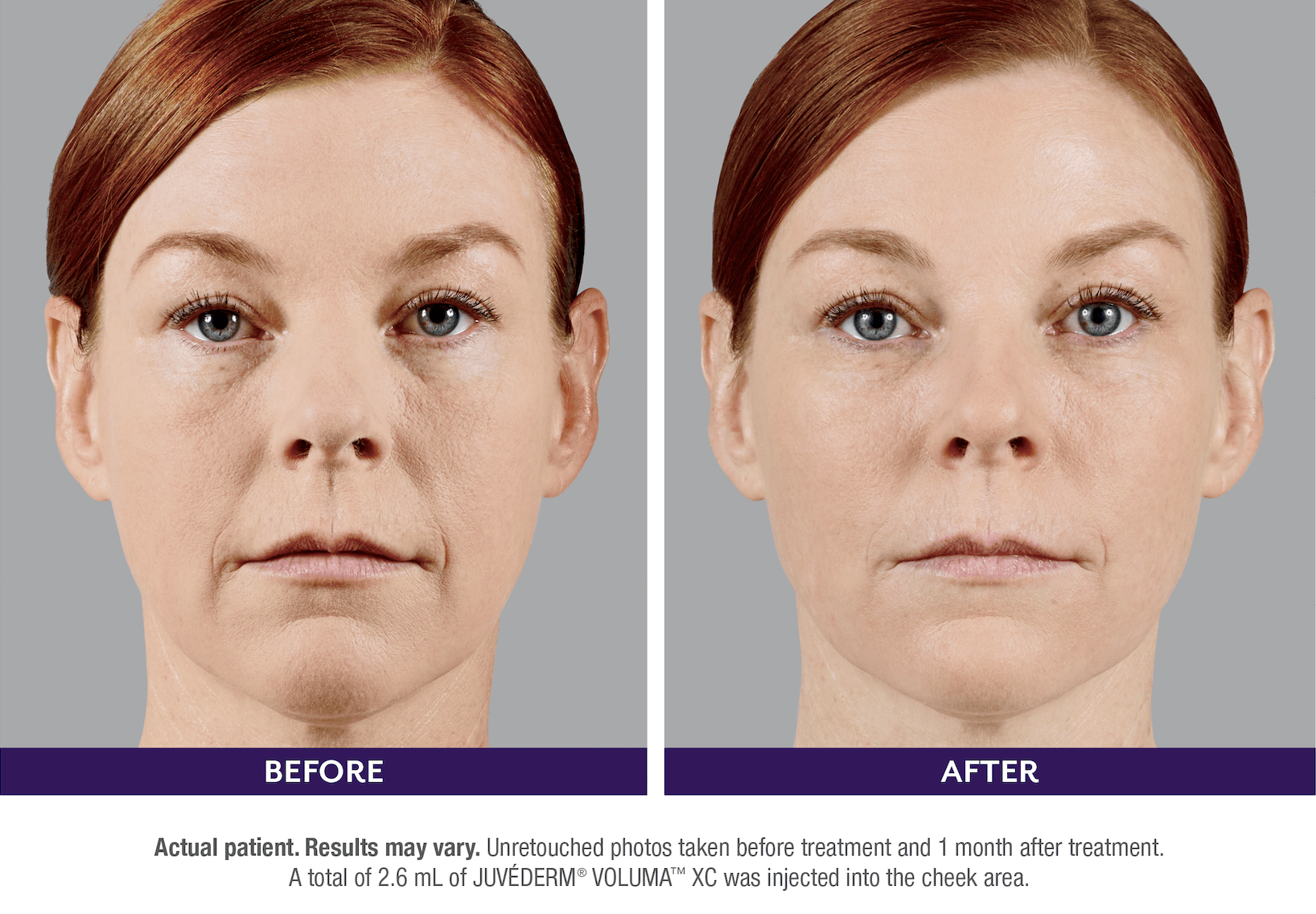
Slide title
Write your caption hereButton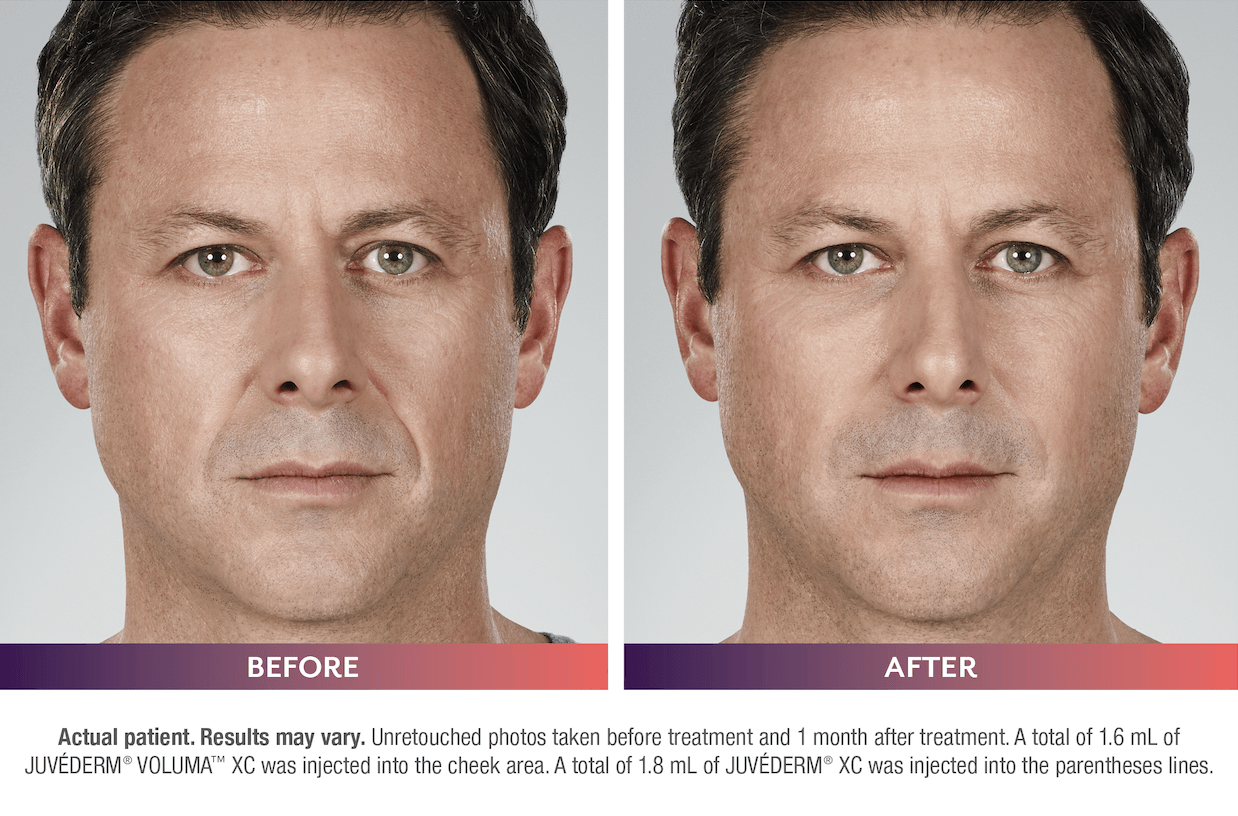
Slide title
Write your caption hereButton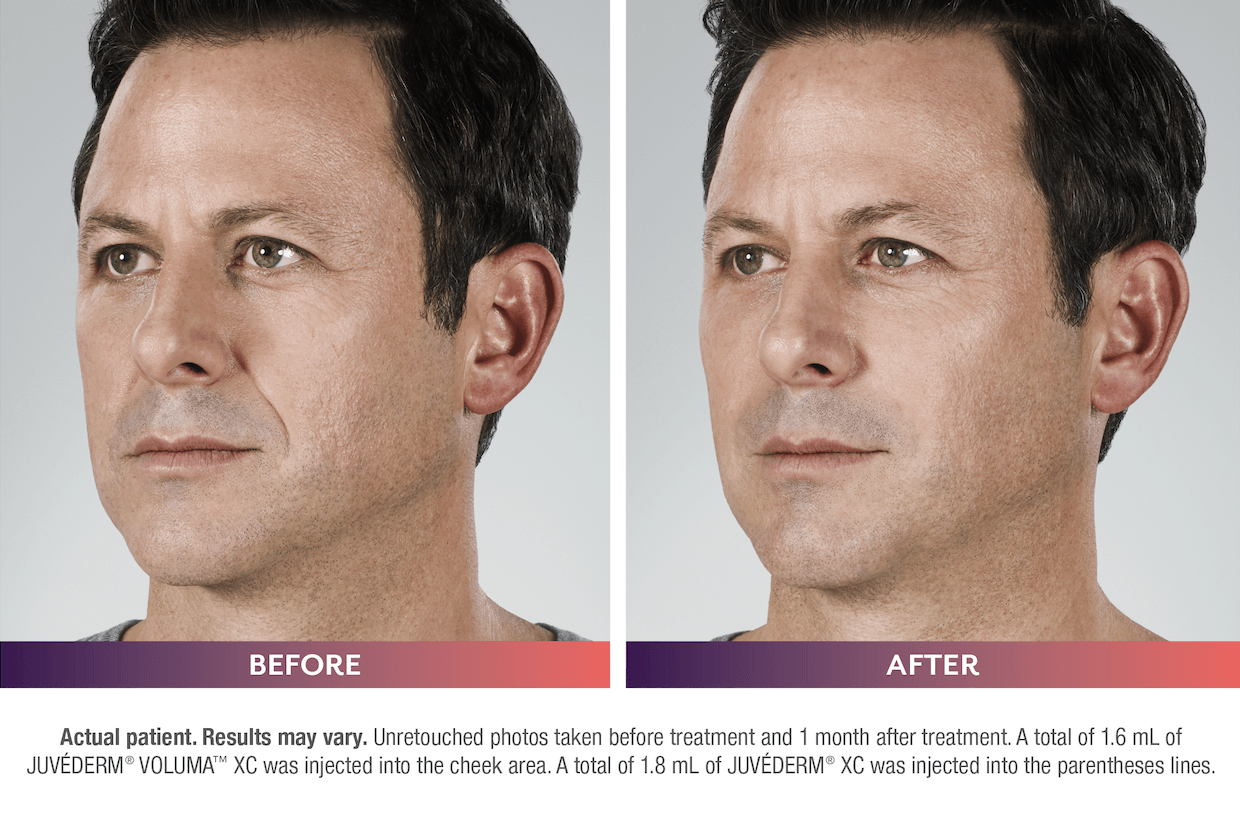
Slide title
Write your caption hereButton
Important Safety Information for JUVÉDERM® & Restylane®
- IMPORTANT SAFETY INFORMATION — JUVÉDERM®
JUVÉDERM® Collection of Fillers Important Information
INDICATIONS
JUVÉDERM® VOLUMA® XC is indicated for deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid-face and for augmentation of the chin region to improve the chin profile in adults over the age of 21.
JUVÉDERM® VOLLURE® XC injectable gel is indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds) in adults over the age of 21.
JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC injectable gels are indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds).
JUVÉDERM® VOLBELLA® XC injectable gel is indicated for injection into the lips for lip augmentation and for correction of perioral rhytids in adults over the age of 21.
JUVÉDERM® Ultra XC injectable gel is indicated for injection into the lips and perioral area for lip augmentation in adults over the age of 21.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to Gram-positive bacterial proteins or lidocaine contained in these products.
WARNINGS
Do not inject into blood vessels. Introduction of these products into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur
Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
To minimize the risk of potential complications, these products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy and product use in indicated areas
The potential risks of soft-tissue injections should be discussed with patients prior to treatment to ensure they are aware of signs and symptoms of complications
The safety and effectiveness for the treatment of anatomic regions other than the mid-face, chin, and prejowl sulcus regions with JUVÉDERM® VOLUMA® XC; facial wrinkles and folds with JUVÉDERM® VOLLURE® XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC; and the lips and perioral area with JUVÉDERM® VOLBELLA® XC and JUVÉDERM® Ultra XC have not been established in controlled clinical studies
The safety for use of these products during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied
The safety for use of JUVÉDERM® VOLUMA® XC has been established in patients between 35 and 65 years of age in cheek augmentation and for patients between 22 and 80 years of age for chin augmentation
The safety for use of JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC in patients under 18 years, and JUVÉDERM® VOLLURE® XC and JUVÉDERM® VOLBELLA® XC in patients under 22 years, has not been established
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection
Use dermal fillers with caution in patients on immunosuppressive therapy
Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites
Patients who experience skin injury near the site of implantation may be at a higher risk for adverse events
The safety for use of JUVÉDERM® VOLUMA® XC injectable gel in patients with very thin skin in the mid-face has not been established
The safety of JUVÉDERM® VOLUMA® XC with cannula for cheek augmentation has not been established in patients with Fitzpatrick Skin Types V and VI
JUVÉDERM® VOLUMA® XC was not evaluated in subjects with significant skin laxity of the chin, neck, or jaw in the chin augmentation study
The effect of JUVÉDERM® VOLUMA® XC injection into the chin on facial hair growth has not been studied
Patients may experience late onset nodules with use of dermal fillers including JUVÉDERM® VOLUMA® XC
Patients may experience late onset adverse events with use of dermal fillers
ADVERSE EVENTS
The most commonly reported side effects for JUVÉDERM® injectable gels were redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA® XC, dryness was also reported. The majority were mild or moderate in severity. For JUVÉDERM® VOLUMA® XC, most resolved within 2 to 4 weeks. For JUVÉDERM® VOLLURE® XC, JUVÉDERM® Ultra Plus XC, or JUVÉDERM® Ultra XC, most resolved within 14 days; and for JUVÉDERM® VOLBELLA® XC, most resolved within 30 days.
To report an adverse reaction with any product in the JUVÉDERM® Collection, please call Allergan at 1-800-433-8871. Please visit JuvedermDFU.com for more information.
Products in the JUVÉDERM® Collection are available only by a licensed physician or properly licensed practitioner.
- IMPORTANT SAFETY INFORMATION - RESTYLANE®
Important Safety Information
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Kysse, Restylane® Refyne, Restylane® Defyne, and Restylane® Contour.
APPROVED USES
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds. Restylane® Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild to moderate chin retrusion.
Restylane® Contour is for cheek augmentation and for the correction of midface contour deficiencies.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To report a side effect with any Restylane® product, please call Galderma Laboratories, L.P at 1-855-425-8722.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
Look like the best version of you
Ready to enhance your unique beauty? Call 615-880-9500 or click the button below to schedule a consultation with our expert Facial Plastic Surgeon, Dr. Ashley Guthrie.
View Real Patient Transformations
Visit Dr. Guthrie's Before & After Gallery to see real patient transformations for our most popular non-surgical treatments.
Guthrie Facial Plastic Surgery | All Rights Reserved
Privacy Policy | Website by Infinity Medical Marketing
Privacy Policy | Website by Infinity Medical Marketing